For the first time, scientists have measured the X-ray signature of a single atom. A multi-center team with contributors from Ohio University, Argonne National Laboratory, the University of Illinois-Chicago, and others published the work in Nature. This study could have enormous implications for scientists’ capability to detect chemical elements in materials.
Trapped at attogram
X-rays are employed in many fields–from scanning broken bones to exposing security threats at airports. When used for scientific research, they help examine materials’ properties. Improvement in analysis instrumentation, such as the development of the X-ray synchrotron, reduces the sample size required for accurate reading. At present, the minimum amount of matter that can be subjected to X-ray analysis is an attogram, which is approximately more than 10 thousand atoms. This is a dramatic step forward in detection.
The weak characteristic X-ray signals that smaller numbers of atoms produce have so far been the limiting factor. “We’ve been looking all along for ways to image systems containing fewer atoms,” said Saw Wai Hla, a scientist at Argonne National Laboratory and physics professor at Ohio University. “Atoms can be routinely imaged with scanning probe microscopes, but without X-rays, you cannot tell what they are made of”” Recently, he added: “Now we can determine exactly what type it is one atom at a time and simultaneously measure its chemical state.”
To overcome this physical barrier, Hla et al. used a custom-built X-ray synchrotron located within the Center for Nanoscale Materials (CNM) at Argonne National Laboratory (ANL).
Elemental fingerprints
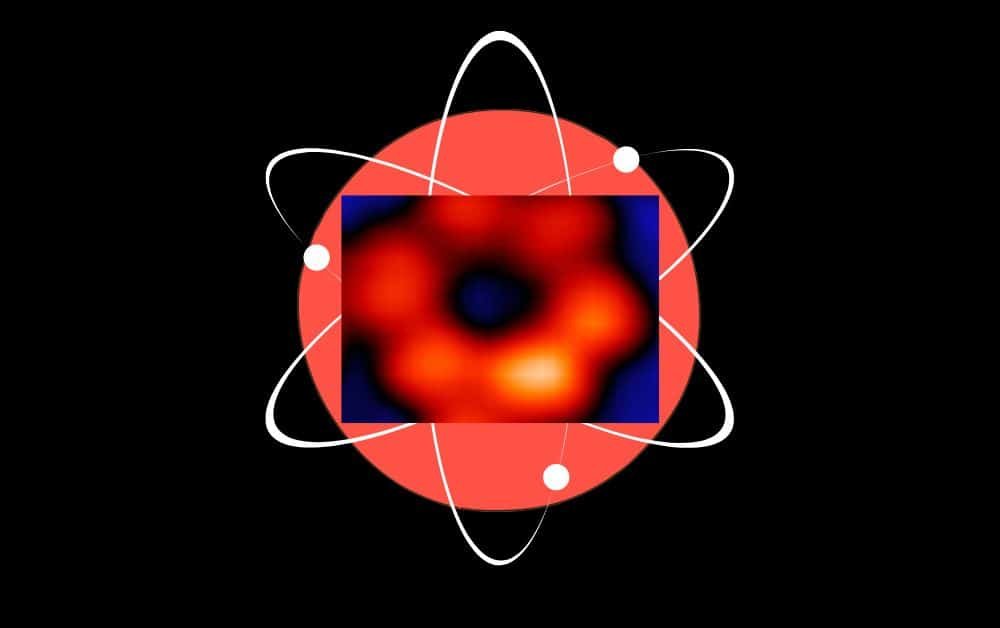
To demonstrate their method, the authors characterized radiation patterns arising from an iron atom and a terbium atom. The authors equipped classical X-raying facilities with a specific detector system based on conventional design principles, including a special rod made from hard metal situated directly near the object being studied.
The rod, being in close proximity to the sample, captured excited electrons generated during the scan. Experts call this method Spectroscopic Synchrotron Scanning Tunnelling Microscopy (SX-STM). Atoms’ energy levels are characteristic of their core physical attributes, thereby serving as unique elemental “fingerprint”s” that enables atom identification.
Fig. 3. When X-rays (blue) illuminate an iron atom (the red ball at the centre of the molecule), core-level electrons are excited. Subsequent tunnelling of x-ray excited electron to the detector apex (grey) takes place via overlapping atomic and molecular orbitals, which provide elemental and chemical information about the iron atom in this case. Credit: Saw-Wai Hla
“The technique and concept we used in this study have proven to be revolutionary for X-ray science and nanoscale studies,” states Tolu” ope Michael Ajayi, a PhD student and one of the study’s authors. “This could”lead to breakthrough discoveries in research using x-rays to detect or understand single atoms, potentially paving the way for new technology such as quantum information processing or ultra-trace analysis for environmental and medical.” The study added: “Moreover, “these achievements pave the way towards modern materials science instrumentation.”
A rare finding
They then characterized how these atoms behaved upon inclusion into different molecular hosts. “Our observ”tions indicated that terbium atom, a rare-earth metal, remained almost completely isolated without change in its chemical state, but iron changed dramatically with its environment,” he stat”d. Hla explained.
Many fields have applications for this brand-new knowledge. Terbium, a rare earth metal used in common appliances and advanced technologies, now allows scientists to observe how its chemical properties change with environmental conditions. This discovery opens up more utilization possibilities.
Moreover, the team developed an innovative methodology known as X-ray excited resonance tunnelling (X-ERT). This technique uses synchrotron X-rays to identify single molecular orbital orientations on a material surface.